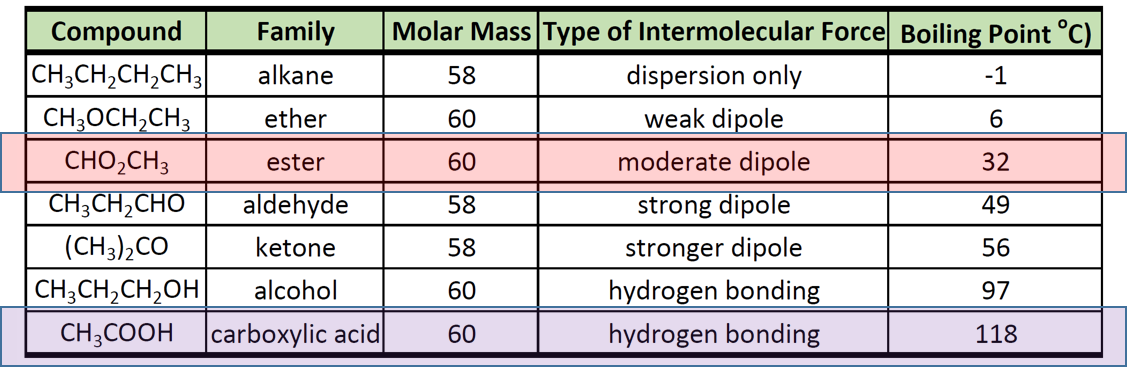
Boiling Point of Ester and Carboxylic Acid
AT b c d and k. All acid derivatives break from RCO.

Solubility And Boiling Point Flashcards Quizlet
Aspirin the ester of salicylic acid is prepared from acetic.
. Why do you wash the dichloromethane solution of your reductive amination product with sodium bicarbonate rather than dilute aqueous HCl. Orthophosphoric Acid H3PO4 Phosphoric Acid - Orthophosphoric acid H3PO4 is one of the most widely used chemicals. Click Here to see full-size table Carboxylic acid derivatives have varied applications.
AT b and k. Students could identify an ester by measuring its boiling point followed by hydrolysis to form the carboxylic acid which is purified by recrystallisation and determine its melting point. Aliphatic ketones give ester.
RCO-O-COR on hydrolysis give carboxylic acids. 458 K Solubility in water. Valeric acid reacts as a typical carboxylic acid.
For example in addition to its use as a disinfectant formic acid the simplest carboxylic acid is employed in textile treatment and as an acid reducing agent. Students could make biodiesel. The general molecular formula for dicarboxylic acids can be written as HO 2 CRCO 2 H where R can be aliphatic or aromatic.
Aldehydes and ketones have lower boiling point than those of alcohols of similar molecular. 497 g100 mL Acidity pK a. Acetic acid is extensively used in the production of cellulose plastics and esters.
Students could make soap. In general dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acidsDicarboxylic acids are also used in the preparation of. A dicarboxylic acid is an organic compound containing two carboxyl functional groups COOH.
Like carboxylic acids phosphoric acid can dimerize via a dehydration reaction to form phospho anhydrides. 185 C 365 F. A Sodium bicarbonate is a good method of removing aldehydes from organic solventb The amine product will be protonated by acid and remain in the aqueous layer as a saltc Sodium bicarbonate transfers the amine starting.
It can form amide ester anhydride and chloride derivatives. Iv From nitriles and amides Nitriles are hydrolysed to amides and then to acids in the presence of Hor OH-as catalyst. Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH 3 CH 2.
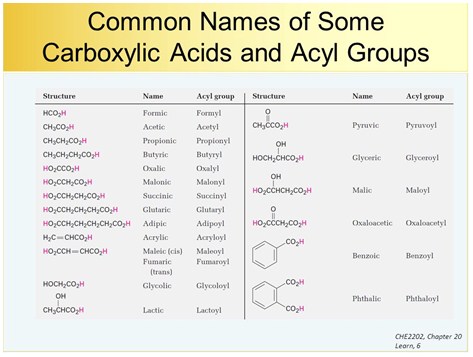
Carboxylic Acids And Esters A Level Chemistry Revision Notes
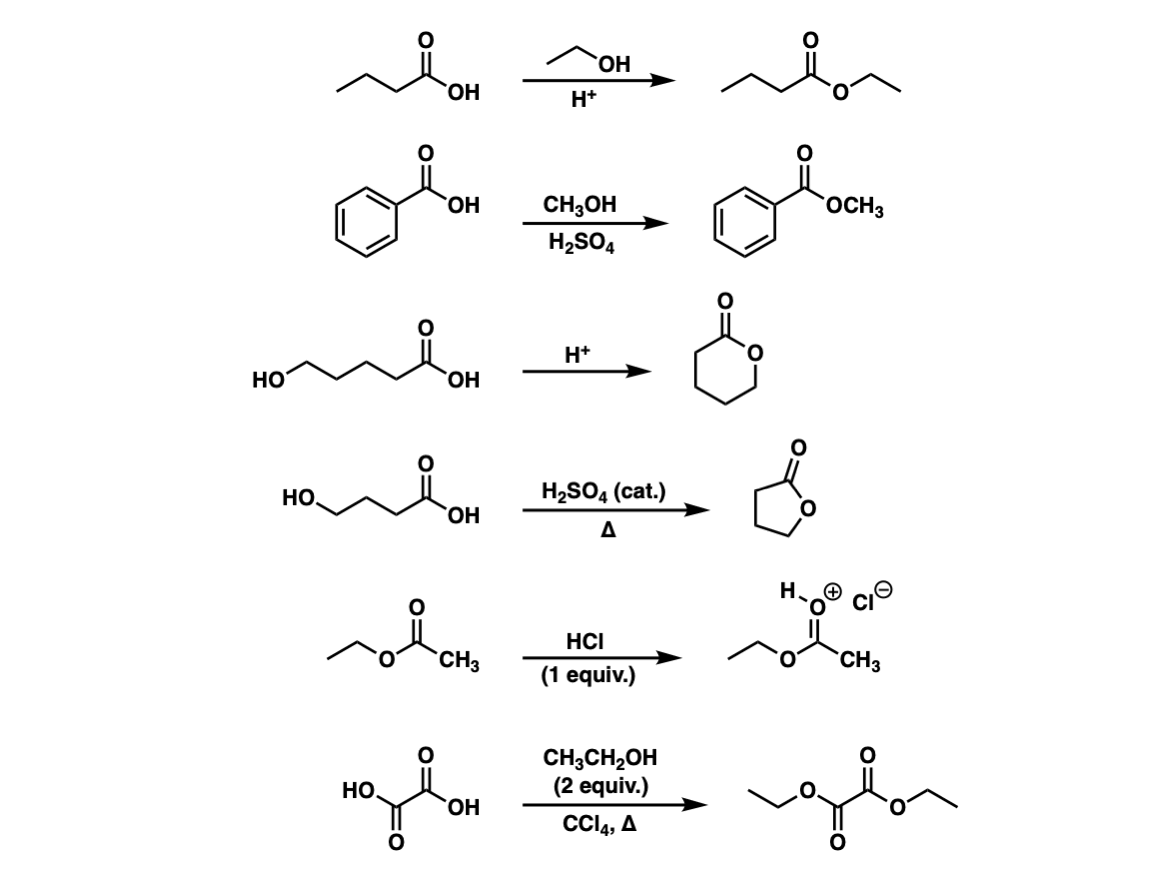
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry
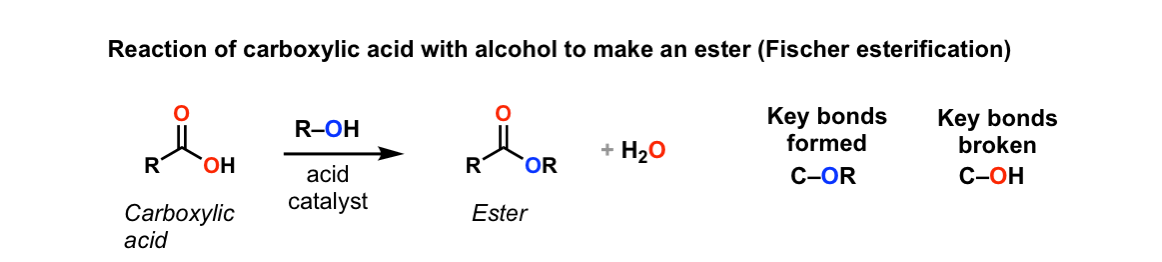
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry

Lesson Explainer Properties Of Esters Nagwa

Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry

Physical Properties Of Carboxylic Acids Youtube
0 Response to "Boiling Point of Ester and Carboxylic Acid"
Post a Comment